Abstract
Background Successful treatment with tacrolimus (TAC) to prevent graft versus host disease (GVHD) and minimize TAC-related toxicities among allogeneic hematopoietic stem cell transplantation (alloHSCT) recipients is contingent upon achieving and maintaining plasma trough concentrations within a narrow therapeutic range. Despite standardized weight-based dosing, inter-individual variability is observed in TAC trough concentrations which may in part be attributable to pharmacogenetic variants influencing the pharmacokinetic disposition of TAC. The primary objective was to investigate the association between CYP3A4, CYP3A5, or ABCB1 genotype and the proportion of patients that attained an initial TAC trough concentration in the therapeutic range following initiation of intravenous (IV) TAC and conversion to oral (PO) TAC administration. Additional associations with clinical outcomes were also explored.
Methods We retrospectively evaluated 86 patients who underwent HLA-matched (8/8) related donor alloHSCT and were prescribed a TAC-based regimen for GVHD prophylaxis between January 1, 2014 and February 28, 2020 at the Moffitt Cancer Center. Data were extracted from the Moffitt BMT Research & Analysis Information Network (BRAIN) database. Patients received TAC in combination with either sirolimus (SIRO), methotrexate (MTX), or other immunosuppressant regimen. Ideal body weight was used to dose TAC unless it was less than the patient's actual body weight. When given with SIRO, TAC targeted trough concentrations were 3 to 7 ng/ml. In patents receiving TAC plus either MTX or other regimens, the target therapeutic range was 10 to 15 ng/ml. Biobanked pre-transplant blood samples were used for CYP3A4/5 and ABCB1 genotyping. Based on the frequency of phenotypes observed, analyses were performed comparing CYP3A5 normal/intermediate (NM/IM) metabolizers to CYP3A5 poor metabolizers (PM), CYP3A4 rapid metabolizers (RM) to CYP3A4 NM/IM/PM, and ABCB1 normal function (NF) to ABCB1 intermediate/low function (IF/LF).
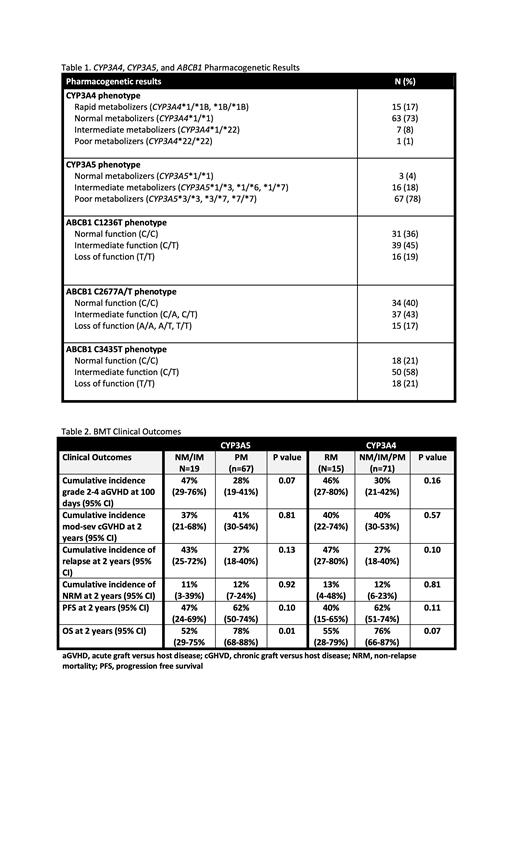
Results Median age at time of alloHSCT was 57 years (range: 20.4-76.7); 60% were men and 83% were white. CYP3A4/5 and ABCB1 phenotypes observed in the study population are presented in Table 1. No significant associations were identified between CYP3A4, CYP3A5, or ABCB1 phenotype groups and the proportion of patients attaining initial therapeutic trough concentrations after the start of IV TAC. In transitioning from IV to PO TAC, 66 of 86 patients had evaluable data. Compared to CYP3A5 PM, CYP3A5 NM/IM were significantly less likely to attain an initial target trough concentration in the therapeutic range following PO TAC administration (40% CYP3A5 NM/IM vs 76.5% CYP3A5 PM, p=0.02). A significantly lower proportion of CYP3A4 RM attained initial target trough concentrations in the therapeutic range following the switch to PO TAC compared to CYP3A4 NM/IM/PM (43% CYP3A4 RM vs 75% CYP3A4 NM/IM/PM, p=0.049). No associations were identified with PO TAC trough concentrations and ABCB1 phenotype groups. The cumulative incidences of grades 2-4 acute GVHD (aGVHD)at day 100 among CYP3A5 NM/IM vs CYP3A5 PM were 47% and 28%, respectively (p=0.07), and for CYP3A4 RM vs CYP3A4 NM/IM/PM were 46% and 30%, respectively (p=0.16). No significant differences were seen in the incidences of chronic GVHD (cGVHD) nor in non-relapse mortality. Relapse rates at 2 years were not significantly higher among patients that were CYP3A5 NM/IM and CYP3A4 RM compared to CYP3A5 PM and CYP3A4 NM/IM/PM, respectively. Overall survival (OS) for CYP3A5 NM/IM was 52% and for PM was 78% (p=0.01). When comparing CYP3A4 groups, OS for RM was 55% and for NM/IM/PM was 76% (p=0.07) (Table 2).
Conclusion The findings of the present study revealed that CYP3A4/5 genotype may play an important role in dosing of PO TAC in alloHSCT recipients, whereas ABCB1 did not significantly influence either route of TAC administration. CYP3A4/5 genotypes may also influence long term survival after transplant. Larger prospective studies are needed to confirm the impact of these genes on GVHD, relapse and survival.
Perkins: AcroTech Pharma: Research Funding. Nishihori: Karyopharm: Research Funding; Novartis: Research Funding. Bejanyan: Magenta: Consultancy, Membership on an entity's Board of Directors or advisory committees; Medexus: Consultancy, Membership on an entity's Board of Directors or advisory committees; American Well Corp (Spouse disclosure): Current equity holder in publicly-traded company; Avrobio (Spouse disclosure): Current equity holder in publicly-traded company; Crispr Therapeutics (Spouse disclosure): Current equity holder in publicly-traded company; Humanigen (Spouse disclosure): Consultancy, Membership on an entity's Board of Directors or advisory committees; Kadmon (Spouse disclosure): Consultancy; Merck (Spouse disclosure): Current equity holder in publicly-traded company; Organon (Spouse disclosure): Current equity holder in publicly-traded company; Teladoc Health (Spouse disclosure): Current equity holder in publicly-traded company; Thermo Fisher (Spouse disclosure): Current equity holder in publicly-traded company; Unitedhealth Group (Spouse disclosure): Current equity holder in publicly-traded company. Pidala: Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Clinical trial support; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Clinical trial support; Regeneron: Consultancy; Incyte: Consultancy; Pharmacyclics: Other: Clinical trial support, Research Funding; BMS: Other: Clinical trial support, Research Funding; Novartis: Other: Clinical trail support; Takeda: Other: Clinical trail support; Jannssen: Other: Clinical trial support; Johnson and Johnson: Other; AbbVie: Other; BMS: Other.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal